
07 Jan What Did My Patient Actually Take? An Overview of Cannabinoids and Semi-Synthetic Cannabinoids
Cannabinoids are classified into three categories based on origin and method of production: natural, semi-synthetic, and synthetic. Natural cannabinoids, such as cannabidiol (CBD) and delta-9 THC, occur abundantly in the cannabis plant.1,2 In contrast, cannabinoids like delta-8 THC, delta-10-tetrahydrocannabinol (delta-10-THC), and hexahydrocannabinol (HHC) can originate through two distinct pathways. These cannabinoids may occur naturally in trace amounts within the cannabis plant, either through biosynthesis or as degradation products of other cannabinoids, or they may be synthesized chemically by modifying hemp-derived CBD, which classifies them as semi-synthetic. Regardless of origin, they are commonly referred to as semi-synthetic cannabinoids.3,4,5,6 Therefore, detecting delta-8 THC, delta-10 THC, or HHC, or their metabolites in biological specimens does not necessarily indicate the use of lab-produced, semi-synthetic cannabinoid products. Lastly, fully synthetic cannabinoids are created entirely in laboratories without plant-based starting material, and their presence in biological specimens does indicate the use of a synthetic cannabinoid.7
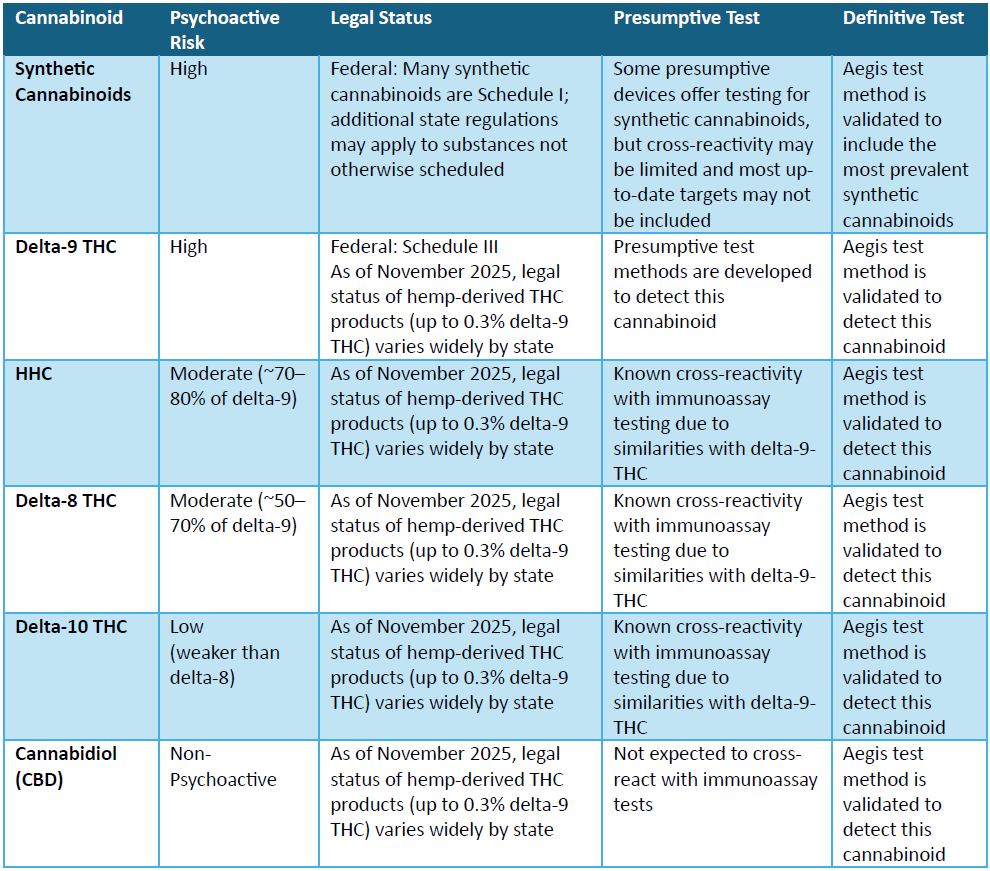
The Agriculture Improvement Act of 2018 (commonly referred to as the 2018 Farm Bill) removed industrial hemp from the federal definition of marijuana. Since then, cannabis has been understood as either hemp (< 0.3% delta-9-THC), used for industrial purposes (paper, rope, and clothing, etc.), or marijuana, a Schedule III controlled substance. The reclassification of marijuana from Schedule I to Schedule III in December 2025 authorized FDA medical research but did not legalize the substance. However, a loophole in the 2018 Farm Bill allowed CBD extracted from hemp to be legally converted into novel semi-synthetic psychoactive cannabinoids, such as delta-8 THC.8 This opened the door for the hemp industry to produce psychoactive semi-synthetic cannabinoids, including HHC and delta-10-THC, without federal regulation.3,9 In November 2025, legislation was passed banning most hemp-derived semi-synthetic cannabinoid products, though it will not take effect until November 2026, leaving room for amendments.4 Until then, these products remain widely available and can produce psychoactive effects similar to delta-9 THC,11 including euphoria, altered perception, cognitive impairment, anxiety, paranoia, hallucinations, psychotic symptoms, and cannabis-induced psychosis (CIP),3,12 as well as an increased risk of psychotic disorders later in life.13 The psychosis risk of cannabinoids can be found in the chart below.
- Di Forti M, Quattrone D, Freeman TP, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry. 2019;6(5):427-436. doi:10.1016/S2215-0366(19)30048-3.
- Kruger J, et al. Delta-8 THC and psychosis: Case reports and clinical observations. J Subst Abuse Treat. 2023;150:108-115.
- Papazisis G, et al. Hexahydrocannabinol (HHC) and psychotic illness: Emerging case series. Psychopharmacol Bull. 2024;54(2):45-52.
- Zuardi AW, et al. Cannabidiol as a potential antipsychotic: Clinical trial evidence. Curr Pharm Des. 2012;18(32):5131-5140.
- ElSohly MA, et al. Chemical and pharmacological differences among THC isomers (delta-8, delta-9, delta-10). J Nat Prod. 2021;84(6):1735-1743.
Aegis has expanded its cannabinoid analysis to include HHC and delta-10 THC, complementing the existing testing for CBD, delta-8-THC, and delta-9-THC. This update provides a focused review of HHC and delta-10 THC, while detailed information on CBD, delta-8-THC, and delta-9-THC can be accessed at https://www.aegislabs.com/clinical-update/thc-delta-8-9-cbd/. Fully synthetic cannabinoids are available for testing under our Novel Psychoactive Substances (NPS) offerings: (https://www.aegislabs.com/services/novel-psychoactive-substances/).
HHC
Hexahydrocannabinol (HHC) is a psychoactive cannabinoid that has recently emerged in commercial cannabis products. While it occurs naturally in the cannabis plant, its presence is limited to trace amounts formed through biosynthesis or as a breakdown product of other cannabinoids. HHC can also be produced through chemical modification of hemp-derived CBD, a process that categorizes it as a semi-synthetic cannabinoid.3,6 Structurally, HHC exists in two isomeric forms, 9(R)-HHC and 9(S)-HHC, distinguished by the orientation of the methyl group at the C9 position. The 9(R) isomer is the active form exhibiting stronger binding to CB1 and CB2 receptors, making it more likely to produce psychoactive effects, whereas 9(S) is considerably less active. HHC is generally considered slightly less potent than delta-9 THC and may be associated with fewer anxiety-related effects.14,15,16 Current pharmacological insights are based largely on receptor studies and animal models,17,18 while human data remain scarce, leaving gaps in knowledge about dosing, toxicity, and long-term outcomes.3
Commercial HHC products vary widely in isomer ratios. An analysis of more than 60 products revealed striking variability in isomer ratios, ranging from 0.2:1 (far less R than S) to 2.4:1 (much more R than S). Because of this variability, predicting effects or risks is difficult. Products rich in the R isomer may have strong psychoactive effects, while those with more of the S isomer may have weaker activity. Given HHC’s emerging status and inconsistent purity, extrapolation to all HHC use should be made with caution.16,19
Delta-10 THC
Far less is known about delta-10 THC due to limited literature. Delta-10 THC occurs naturally in cannabis mainly as a degradation product of other cannabinoids, but it can also be synthesized from hemp-derived CBD—a process that designates it as a semi-synthetic cannabinoid.4,5 Like HHC, delta-10 THC exists in two stereoisomeric forms—(6aR,9R)-Delta-10 THC and (6aR,9S)-Delta-10 THC, which differ in their spatial arrangement at the C9 position. The R isomer is believed to interact with CB1 receptors and may produce psychoactive effects, while the S isomer produces little to none.20,21 Delta-10 THC is believed to be slightly less potent than delta-8 THC, a conclusion drawn primarily from receptor-binding mechanisms due to the lack of robust clinical data. It is plausible that consumers may encounter varying isomer mixes, yet no studies have quantified these differences or their clinical implications.
Testing
Aegis performs initial presumptive testing, followed by reflex definitive analysis using mass spectrometry to differentiate CBD, delta-9 THC, delta-8 THC, HHC isomers, delta-10 THC isomers, and their associated metabolites.
Other definitive cannabinoid assays that test only for delta-9 THC are no longer adequate to address this emerging public safety concern.22 Lastly, if immunoassay testing is used alone, results should be interpreted with caution. These screens primarily detect delta-9 carboxy-THC, but structural similarities with delta-10 THC and HHC compounds can lead to cross-reactivity, increasing the risk of false positives or misinterpretation.23,24
Oral Fluid
Aegis is updating our oral fluid cannabinoid test (previously listed as Marijuana) to include cannabidiol (CBD), delta-8 THC, delta-10 THC, and HHC. Reports for oral fluid testing will now specify the exact cannabinoid detected, and delta-9 THC will no longer be reported as Marijuana. Since Delta-10 THC and HHC exist in multiple isomeric forms, both isomers will be included (see chart below). Parent drug is typically present at much higher concentrations than metabolites in oral fluid, so metabolites will not be included in the oral fluid analysis.25
ORAL FLUID
0440OF Cannabinoids (Marijuana)
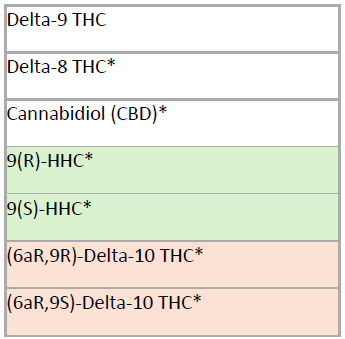
*Newly added
Cannabinoid detection in oral fluid is largely due to direct contamination of the mouth during smoking or vaping, not systemic exposure. This “depot effect” means oral fluid tests are more likely to detect smoked or vaporized cannabinoids shortly after use than ingested edibles or medications.26,27,28 For example, individuals using dronabinol (Marinol®) are unlikely to test positive at Aegis’s 2 ng/mL threshold, while those who smoke or vape may test positive shortly after use.29 This makes oral fluid useful for distinguishing smoked cannabis from oral cannabis medication when interpreting positive results.
Urine
After ingestion, delta-9 THC undergoes rapid and extensive hepatic metabolism, and only a negligible amount (<1%) of parent delta-9 THC is excreted unchanged in urine. Its metabolites, particularly delta-9 carboxy-THC, are the most reliable indicators of prior delta-9 THC exposure. Semi-synthetic cannabinoids such as delta-8 THC, delta-10 THC, and HHC are similarly extensively metabolized.19,22 Aegis now includes HHC metabolites (9(R)-Carboxy-HHC and 9(S)-Carboxy-HHC) and the delta-10 THC metabolite, (delta-10-carboxy-THC), in our updated urine cannabinoid testing panel (see chart below).
URINE (Marijuana 04440):
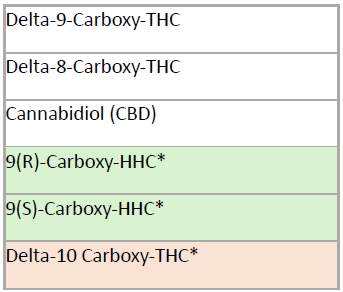
*Newly added
Urinary elimination of delta-9 carboxy-THC has been studied extensively, but data on newer semi-synthetic cannabinoids remain limited. Due to structural similarities, their detection windows may resemble that of delta-9 THC.9
Many factors influence detection periods and prevent a mathematical or direct correlation between drug dose and urine concentration. They can include the dose, timing of dose relative to specimen collection, frequency of dose leading up to the time of collection, chronicity of use, body composition, hydration (indicated by creatinine levels), extent of drug absorption, liver or kidney function, drug interactions, and genetic variations that affect drug metabolism.30,31,32 Chronic heavy users and individuals with higher adipose tissue may have longer detection periods.33
Summary
Even with advanced testing capable of differentiating specific cannabinoids, results cannot confirm the source of the cannabinoid. For instance, many CBD products marketed as “THC-free” may contain trace amounts of delta-9 THC or other cannabinoids because of poor manufacturing controls or mislabeling.34,35 Similarly, natural THC products may include small amounts of degradants such as delta-8 THC, HHC isomers, or delta-10 THC isomers.3,4,5,6 Because of factors like elapsed time since use, potential multiproduct consumption, and unknown product composition, testing alone cannot definitively identify the source of a positive result. Monitoring use patterns, adverse events, and toxicity reports remains essential, particularly as psychoactive cannabinoids become more widely available despite limited safety data.3 The inclusion of additional cannabinoids in Aegis testing is intended to support providers in assessing psychoactive cannabinoid use, regardless of origin.
Please call our clinical scientists at 1-877-552-3232 if you require additional information.
NOTICE: The information above is intended as a resource for health care providers. Providers should use their independent medical judgment based on the clinical needs of the patient when making determinations of who to test, what medications to test, testing frequency, and the type of testing to conduct.
References:
- Abdel-Kader MS, Radwan MM, Metwaly AM, et al. Chemistry and pharmacology of delta-8-tetrahydrocannabinol. Molecules. 2024;29(6):1249. doi:10.3390/molecules29061249.
- Wilson, W.B., Romares, A. & Goldman, S. An In-Depth Study Evaluating the Determination of CBD, Δ9-THC, Δ8-THC, and 25 Additional Cannabinoids in Cannabis Concentrates by Liquid Chromatography with Absorbance Detection. Chromatographia (2025). https://doi.org/10.1007/s10337-025-04464-x
- Ujváry I. Hexahydrocannabinol and closely related semi-synthetic cannabinoids: A comprehensive review. Drug Test Anal. 2024;16(2):127-161. doi:10.1002/dta.3519
- Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33(12):1357-1392. doi:10.1039/c6np00074f
- Turner CE, Elsohly MA, Boeren EG. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod. 1980;43(2):169-234. doi:10.1021/np50008a001. Delta-10 THC. ACS Laboratory. Published 2021. Accessed December 15, 2025. https://www.acslab.com/delta-10-thc/
- Grapp M, Kaufmann C, Peschel A, Potzscher M, Dziadosz M, Marquenie L, Teske J. Detection of 11-nor-9-carboxy-hexahydrocannabinol (HHC-COOH) as metabolite of both hexahydrocannabinol (HHC) and Δ9-tetrahydrocannabinol (Δ9-THC) in routine forensic samples. J Pharm Biomed Anal. 2025 Aug 15;261:116833. doi: 10.1016/j.jpba.2025.116833. Epub 2025 Mar 22. PMID: 40139040.
- National Institute on Drug Abuse. Synthetic Cannabinoids. NIDA website. Published 2023. Accessed December 15, 2025. https://nida.nih.gov/research-topics/synthetic-cannabinoids.
- Kafka DC, Shelby MK. The 2018 Farm Bill’s Hemp Definition and Legal Challenges to State Laws Restricting Certain THC Products. Congressional Research Service. August 20, 2025. https://crsreports.congress.gov/product/pdf/R/R48637. Accessed December 3, 2025.
- Patton AL, Karschner EL, Muir L, Schrecker JZ. LC–MS–MS confirmation of 11-nor-9-carboxytetrahydrocannabinol (Δ8, Δ9, Δ10) and hexahydrocannabinol metabolites in authentic urine specimens. J Anal Toxicol. 2025;49(2):96-103. doi:10.1093/jat/bkae091.
- Saul Ewing LLP. Congress Enacts Hemp THC Products Ban — What the New Federal Restrictions Mean for the Industry. JD Supra website. https://www.jdsupra.com/legalnews/congress-enacts-hemp-thc-products-ban-3896521/. Published November 13, 2025. Accessed December 3, 2025
- Johnson L, Malone M, Paulson E, et al. Potency and safety analysis of hemp delta-9 products: the hemp vs. cannabis demarcation problem. J Cannabis Res. 2023;5(1):29. Published 2023 Jul 26. doi:10.1186/s42238-023-00197-6
- Denton EE, Jung SS, Ventura CAI. Clinical Diagnosis and Management of Gradual Onset Cannabis-Induced Psychosis Following the Consumption of Delta-8-Tetrahydrocannabinol. Cureus. 2024;16(3):e55464. Published 2024 Mar 3. doi:10.7759/cureus.55464
- Di Forti M, Quattrone D, Freeman TP, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry. 2019;6(5):427-436. doi:10.1016/S2215-0366(19)30048-3.
- Durydivka O, Palivec P, Gazdarica M, Mackie K, Blahos J, Kuchar M. Hexahydrocannabinol (HHC) and Δ9-tetrahydrocannabinol (Δ9-THC) driven activation of cannabinoid receptor 1 results in biased intracellular signaling. Sci Rep. 2024;14(1):9181. Published 2024 Apr 22. doi:10.1038/s41598-024-58845-7
- Graziano, S., Vari, M. R., Pichini, S., Busardo, F. P., Cassano, T., & Di Trana, A. (2023). Hexahydrocannabinol Pharmacology, Toxicology, and Analysis: The First Evidence for a Recent New Psychoactive Substance.Curr Neuropharmacol, 21 (12), 2424-2430. doi:10.2174/1570159X21666230623104624
- Nasrallah DJ, Garg NK. Studies Pertaining to the Emerging Cannabinoid Hexahydrocannabinol (HHC). ACS Chem Biol. 2023 Sep 15;18(9):2023-2029. doi: 10.1021/acschembio.3c00254. Epub 2023 Aug 14. PMID: 37578929; PMCID: PMC10510108.
- Reggio, P. H., Greer, K. V., & Cox, S. M. (1989). The importance of the orientation of the C9 substituent to cannabinoid activity. Journal of Medicinal Chemistry, 32 (7), 1630-1635. doi:10.1021/jm00127a038
- Russo F, Vandelli MA, Biagini G, et al. Synthesis and pharmacological activity of the epimers of hexahydrocannabinol (HHC). Sci Rep. 2023;13(1):11061. Published 2023 Jul 8. doi:10.1038/s41598-023-38188-5
- Pitterl F, Pavlic M, Liu J, Oberacher H. Insights into the human metabolism of hexahydrocannabinol by non-targeted liquid chromatography-high-resolution tandem mass spectrometry. J Anal Toxicol. 2024;48(5):350-358. doi:10.1093/jat/bkae022
- Haghdoost M, Brumar D, Geiling B, Brunstetter M, Bonn-Miller MO. Chemistry, crystal structure, and in vitro receptor binding of Δ10-THC isomers. Cannabis Cannabinoid Res. 2023;8(Suppl 1):S45-S56. doi:10.1089/can.2023.0045.
- World Health Organization Expert Committee on Drug Dependence. Tetrahydrocannabinol (isomers of THC): critical review. WHO Technical Report Series. 2018. Available from: https://www.jstor.org/stable/pdf/resrep47919.15.pdf
- Muir LS, Doumit SE, Seither JZ, Knittel JL, Walterscheid JP, Karschner EL. Comprehensive LC-MS-MS analysis of THC isomers, analogs, homologs, and metabolites in blood and urine. J Anal Toxicol. 2025 May 16;49(5):322-331. doi: 10.1093/jat/bkaf023. PMID: 40098275.
- Wolf CE, Pokhai AA, Poklis JL, Williams GR. The cross-reactivity of cannabinoid analogs (delta-8-THC, delta-10-THC and CBD), their metabolites and chiral carboxy HHC metabolites in urine of six commercially available homogeneous immunoassays. J Anal Toxicol. 2023;47(8):732-736. doi:10.1093/jat/bkad059.
- Moody MT, Ringel MM, Mathews CM, Midthun KM. Determination of cross-reactivity of contemporary cannabinoids with THC direct immunoassay (ELISA) in whole blood. J Anal Toxicol. 2022;46(8):844-851. doi:10.1093/jat/bkac051.
- Lin L, Amaratunga P, Reed J, Huang P, Lemberg BL, Lemberg D. Quantitation of Δ8-THC, Δ9-THC, cannabidiol and 10 other cannabinoids and metabolites in oral fluid by HPLC–MS-MS. J Anal Toxicol. 2022;46(1):76-88. doi:10.1093/jat/bkaa184.
- Huestis, M. A., & Smith, M. L. (2023). Cannabinoid detection in oral fluid: Depot effect and interpretation challenges. Journal of Analytical Toxicology, 47(2), 85–94. https://doi.org/10.1093/jat/bkad012
- Milman G, Barnes AJ, Schwope DM, et al. Disposition of cannabinoids in oral fluid after controlled around-the-clock oral THC administration. Clin Chem. 2010;56(8):126-9.
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2020). Clinical drug testing in primary care: Technical assistance publication (TAP 32). Rockville, MD: U.S. Department of Health and Human Services. https://store.samhsa.gov/product/TAP-32-Clinical-Drug-Testing-in-Primary-Care/SMA12-4668
- Newmeyer et al., 2014. Cannabinoid Disposition in Oral Fluid after Controlled Cannabis Smoking in Frequent and Occasional Smokers. Drug Test Anal. 2014 October ; 6(10): 1002–1010. doi:10.1002/dta.1632.
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2020). Clinical drug testing in primary care: Technical assistance publication (TAP 32). Rockville, MD: U.S. Department of Health and Human Services. https://store.samhsa.gov/product/TAP-32-Clinical-Drug-Testing-in-Primary-Care/SMA12-4668
- Moeller KE, Kissack JC, Atayee RS, Lee KC. Clinical interpretation of urine drug tests: What clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774–796.
- Langman, L. J., & Bechtel, L. K. (2018). Clinical toxicology testing: A guide for laboratory and medical professionals. Journal of Applied Laboratory Medicine, 3(4), 631–644. https://doi.org/10.1373/jalm.2018.027631
- Verstraete AG. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther Drug Monit. 2004;26(2):200–205.
- Spindle T, Vandrey R, et al. Study shows widespread mislabeling of CBD content occurs for over-the-counter products. JAMA Netw Open. Published July 20, 2022. Available from: https://www.hopkinsmedicine.org/news/newsroom/news-releases/2022/07/study-shows-widespread-mislabeling-of-cbd-content-occurs-for-over-the-counter-products
- Gidal BE, Vandrey R, Wallin C, et al. Product labeling accuracy and contamination analysis of commercially available cannabidiol product samples. Front Pharmacol. 2024;15:1335441. doi:10.3389/fphar.2024.1335441


