
16 Oct Opioid Use Disorder: Evaluating Risk of Return to Use
Current Landscape of Opioid Use Disorder (OUD)
Although opioid use patterns vary by drug type, geographic region, and population, reports suggesting a decline in opioid-related mortality can be misleading. The United States is currently experiencing what has been termed the “fourth wave” of the opioid epidemic.1 The fourth wave of the opioid epidemic is defined by the combined use of illicit fentanyl (including fentanyl analogs) with polysubstance use, particularly the rising combination of opioids and stimulants. There is also rising use and detection of non-fentanyl synthetic opioids such as nitazenes. The current crisis is marked by overdose deaths increasingly involving fentanyl mixed with methamphetamine, cocaine, benzodiazepines, alcohol, and gabapentinoids. This polysubstance pattern is especially concerning because it both increases overdose risk and complicates treatment, as medications for opioid use disorder (MOUD) do not address stimulant use disorders. Data shows a sharp increase in stimulant and opioid polysubstance use, with methamphetamine and fentanyl dominating in western states and cocaine and fentanyl combinations more common in the east.1,2 Between 2010 and 2021, overdose deaths involving fentanyl and stimulants rose more than fifty-fold in the United States.3
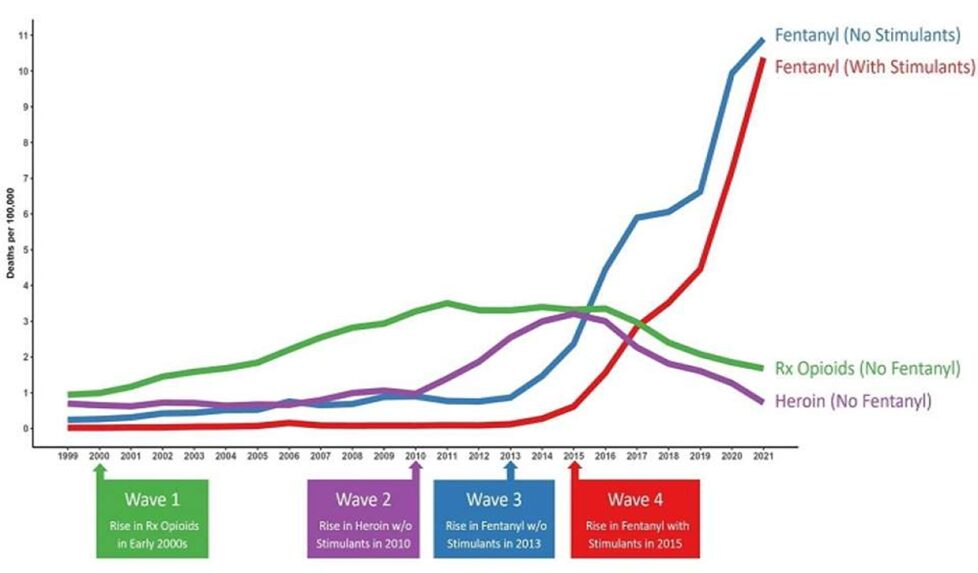
Source: https://www.uclahealth.org/news/release/overdose-deaths-fentanyl-laced-stimulants-have-risen-50-fold32025 NPS order rate by class as % of samples with any NPS class ordered in Q1 and Q2
Approximately half of all overdose deaths now involve both opioids and stimulants, highlighting the need for integrated harm reduction strategies, increased access to naloxone, expanded access to medication-assisted treatment (MAT), and ongoing public health efforts.4-6
Return to Use Risk
Individuals with OUD who do not utilize MAT face return to use rates as high as 80–90%. This high risk of return to use highlights the chronic, relapsing nature of the condition.7 With interventions like buprenorphine and methadone, rates can decline to ~40%.6 MOUD reduce but do not eliminate the risk of return to use.8,9 Despite the benefits of treatment, successful long-term abstinence remains uncommon, a concept that shares similarities with other common chronic diseases (e.g., hypertension, type 2 diabetes). This emphasizes the importance of harm reduction approaches to mitigate the risk of overdose death. Such strategies include early identification and detection of return to use, access to naloxone, and evidence-based treatment programs.10,11
Role of Definitive Testing in Identifying Return to Use12-15
Definitive testing in OUD care is used for monitoring treatment adherence, detecting non-prescribed or illicit substance use, and early identification of return to use. It provides more accurate and comprehensive results than point-of-care (immunoassay) tests, helping clinicians make safer prescribing decisions and tailor interventions. There is evidence that consistent toxicology monitoring improves engagement, supports recovery, and mitigates return to use. Most studies and CDC reports note that careful monitoring is a key element of comprehensive OUD treatment programs. Definitive testing is best understood as a clinical support tool that helps reduce the risk of overdose by:
- Identifying undisclosed use and/or unintentional exposure to illicit substances
- Confirming medication adherence (e.g., buprenorphine, methadone)
- Informing timely interventions if return to use occurs
Aegis Data on Detection of Return to Use
We sought to evaluate return to use by leveraging data with results reported from our laboratory to healthcare providers between 6/1/2024 – 9/30/2025. The primary focus of this analysis was on use, and potential reuse, of fentanyl or fentanyl-containing substances. Included individuals had an initial sample within the defined timeframe and tested positive for fentanyl and/or norfentanyl by definitive testing. Results were excluded if there were any reported samples within the timeframe where 1) an opiate or opioid utilized for pain management was indicated as prescribed or 2) if < 365 days had elapsed between the first and last sample collected per individual or 3) the individual had provided a sample for testing to our laboratory prior to 6/1/2024
In total, there were 402 individuals that met the criteria listed above. Of those meeting inclusion criteria, 367 (91.2%) either had a medication commonly used for the purpose of MAT (i.e., buprenorphine, methadone, or naltrexone) indicated as prescribed at the time of order placement or tested and detected in at least one of their samples included in the analysis. Details regarding select high-risk substances that were requested to be tested and reported as detected when fentanyl was also detected in a sample can be found in the table below.
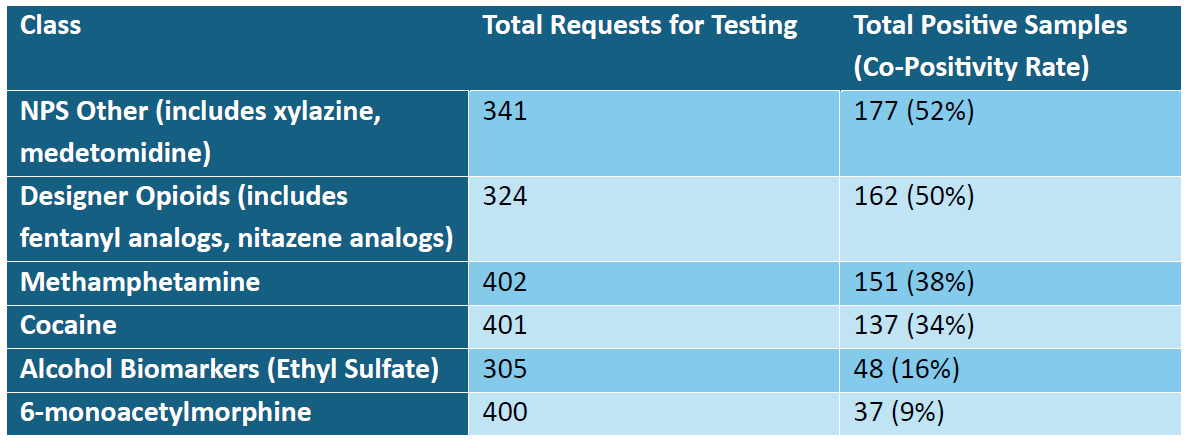
Of those individuals with results that met the criteria, 116 individuals (28.9%) had zero samples reported with negative fentanyl results within the timeframe of the analysis. For those individuals that did have at least one reported result without fentanyl detected (N: 286; 71.1%), additional analyses were performed to identify timeframes for return to use.
We aimed to assess return to use events by analyzing results that were collected and reported to the ordering providers after each individual’s initial result. First, we evaluated longitudinal results for each individual to identify their first sample that did not have fentanyl identified. From there, we defined return to use based on positive findings for fentanyl using the following criteria:
- Positive results for fentanyl or norfentanyl in an oral fluid specimen
- *Presence of parent fentanyl in a urine specimen or
- *Presence of norfentanyl in excess of our established upper limit of quantification for this analyte (1,500 ng/ml).
*Criteria 2 and 3 were included in an attempt to rule out fentanyl detection secondary to protracted elimination after chronic use, a phenomenon that has described in literature for this lipophilic drug.16
Within the results of those 286 individuals that were further analyzed, 101 individuals (35.3%) were identified as having a return to use event based on the criteria established above. Details related to the timing of these events can be found in the table below.

Conclusion
Individuals that use drugs are more frequently encountering substances that contain drugs of multiple classes or are concurrently seeking out drugs of various types. This is supported by data discussing the current phase of the opioid epidemic which is characterized by polysubstance use. Our data supports this notion, demonstrating significant co-positivity in biological samples between illicitly manufactured fentanyl and other drug classes such as illicit adulterants (e.g., xylazine and medetomidine), designer opioids, methamphetamine, and cocaine. As highly adulterated drugs become increasingly pervasive, healthcare providers can utilize testing to gain an improved sense of what drugs individuals seeking treatment have recently encountered.
Opioid use disorder is recognized as a chronic illness with a tendency for relapse. Similar to other long-term health conditions, patients may experience variations in their ability to achieve and sustain treatment objectives over time. It is necessary to both remain aware that return to use can occur as well as equip healthcare providers with the means to effectively identify return to use when it does occur. On average, individuals within the assessed population were assessed as experiencing a return to use between 8-9 months after initiation of treatment which was identified as the date on which their first sample was received at Aegis for testing. We are hopeful the information provided by this analysis not only supports previous publications regarding risk for return to use, but also drives awareness of the chronic nature of OUD.
NOTICE: The information above is intended as a resource for health care providers. Providers should use their independent medical judgment based on the clinical needs of the patient when making determinations of who to test, what medications to test, testing frequency, and the type of testing to conduct.
References:
- Ciccarone D. The rise of illicit fentanyls, stimulants, and the fourth wave of the opioid overdose crisis. Curr Opin Psychiatry. 2021 Jul 1;34(4):344-350.
- Friedman J, et al. Charting the fourth wave: Geographic, temporal, race/ethnicity and demographic trends in polysubstance fentanyl overdose deaths in the United States, 2010–2021. Addiction. 2023;118(12):319-331. https://onlinelibrary.wiley.com/doi/epdf/10.1111/add.16318 Accessed September 19, 2025.
- Friedman J, Shover C. Overdose deaths from fentanyl-laced stimulants have risen 50-fold since 2010. UCLA Health. Published Sept 2022. https://www.uclahealth.org/news/release/overdose-deaths-fentanyl-laced-stimulants-have-risen-50-fold
- Rawson, R. A., Erath, T. G., & Clark, H. W. (2023). The fourth wave of the overdose crisis: Examining the prominent role of psychomotor stimulants with and without fentanyl. Preventive Medicine, 176.
- Park JN, Schneider KE, Fowler D, Sherman SG, Mojtabai R, Nestadt PS. Polysubstance Overdose Deaths in the Fentanyl Era: A Latent Class Analysis. J Addict Med. 2022 Jan-Feb 01;16(1):49-55.
- U.S. Department of Health and Human Services. Harm Reduction. Overdose Prevention Strategy. Accessed September 10, 2025. https://www.hhs.gov/overdose-prevention/harm-reduction
- Bailey GL, Herman DS, Stein MD. Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J Subst Abuse Treat. 2013 Sep;45(3):302-5.
- Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363-371.
- Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017;357.
- Garnett MF, Miniño AM. Drug overdose deaths in the United States, 2003–2023. NCHS Data Brief, no 522. Hyattsville, MD: National Center for Health Statistics; December 2024. Accessed September 10, 2025.
- U.S. Department of Health and Human Services. Harm Reduction. Overdose Prevention Strategy. Accessed September 10, 2025. https://www.hhs.gov/overdose-prevention/harm-reduction
- National Institute on Drug Abuse. Medications for opioid use disorder. National Institutes of Health. Updated June 2021. Accessed September 11, 2025. https://nida.nih.gov/publications/research-reports/medications-to-treat-opioid-addiction
- Jarvis M, Williams J, Hurford M, et al. Appropriate Use of Drug Testing in Clinical Addiction Medicine. J Addict Med. 2017;11(3):163-173.
- Office of the Surgeon General (US). Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Department of Health and Human Services; 2016. Accessed September 11, 2025. https://www.ncbi.nlm.nih.gov/sites/books/NBK574910/pdf/Bookshelf_NBK574910.pdf
- Centers for Disease Control and Prevention. Opioid Use Disorder: Diagnosis. CDC. Accessed September 11, 2025. https://www.cdc.gov/overdose-prevention/hcp/clinical-care/opioid-use-disorder-diagnosis.html
- Celik M, Zimmerer E, Maxwell B, Aloezos C. Challenges of Drug Testing in Addiction Treatment: A Case Report of Protracted Fentanyl Clearance in a Patient Involved With Child Protective Services and Probation. J Addict Med. Published online March 7, 2025.


